JIAOZUO ZHONGWEI SPECIAL PRODUCTS PHARMACEUTICAL CO.,LTD


| Availability: | |
|---|---|
The polyvinylpyrrolidone (Povidone) series, including K15, K17, K25, K30 and K90, are high-quality pharmaceutical excipients that meet EP and USP standards.
They have excellent hygroscopicity, film-forming properties and complexing abilities, making them very suitable for cosmetic, pharmaceutical and industrial applications.
These products are certified to ensure compliance with international quality and safety standards, providing market access and buyer confidence.
Structure formula:
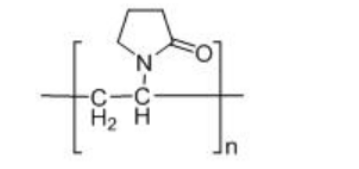
Appearance: White or yellowish-white powder
CDE register number: K30: F20209990878,K90: F20230000040
Application: K25, K30: binders, film formers, dispersers, stabilisers for oral preparation:
K90: binders,thickeners, stabilisers for oral preparation.
PVP K15 | PVP K17 | PVP K25 | PVP K30 | PVP K60 | PVP K90 | PVP K120 | |
pH | 3.0-5.0 | 3.0-5.0 | 3.0-5.0 | 3.0-5.0 | 4.0-7.0 | 4.0-7.0 | 4.0-7.0 |
K-value | 12.75-17.25 | 15.30-18.36 | 24.0-27.0 | 27.0-32.4 | 54.0-64.8 | 81.0-97.2 | 108.0-130.0 |
Aldehydes (ppm) | ≤500 | ≤500 | ≤500 | ≤500 | ≤500 | ≤500 | ≤500 |
Peroxides (ppm) | ≤400 | ≤400 | ≤400 | ≤400 | ≤400 | ≤400 | ≤400 |
Formic acid (%) | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 |
Hydrazine (ppm) | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 |
NVP (ppm) | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
2-Pyrrolidone(%) | ≤3.0 | ≤3.0 | ≤3.0 | ≤3.0 | ≤3.0 | ≤3.0 | ≤3.0 |
Heavy Metals (ppm) | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
Water (%) | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 |
Sulfated ash (%) | ≤0.1 | ≤0.1 | ≤0.1 | ≤0.1 | ≤0.1 | ≤0.1 | ≤0.1 |
Nitrogen (%) | 11.5-12.8 | 11.5-12.8 | 11.5-12.8 | 11.5-12.8 | 11.5-12.8 | 11.5-12.8 | 11.5-12.8 |
All products comply with Ph. Eur, USP/NF, JP,BP certificate of analysis.
Packed in 25 kg plastic drum or fiber drum, inner lined with PE bags. Special packing is available according to customer's requirement. Preserve in tight containers.
A1: Povidone (PVP K-series) is primarily used as a binder, film-former, and disintegrant in oral preparations. It is suitable for a variety of pharmaceutical applications, including tablets, capsules, and granules, ensuring improved dissolution rates and bioavailability of drugs.
A2: Povidone (PVP K15) has a pH range of 3.0 to 5.0, a K value between 12.75 and 17.25, and a water content of less than or equal to 5.0%. It contains less than 500 ppm of aldehydes and peroxides, and less than 1 ppm of hydrazine.
A3: All Povidone (PVP K-series) products comply with Ph. Eur, USP/NF, JP, and BP certificate of analysis, ensuring they meet international quality and safety standards.
A4: Povidone (PVP K-series) is packed in 25 kg plastic drums or fiber drums, inner lined with PE bags. It should be stored in tight containers to maintain its quality and stability.
A5: Povidone (PVP K-series) offers benefits such as improved dissolution rates, enhanced bioavailability, and increased stability of pharmaceutical formulations.
The polyvinylpyrrolidone (Povidone) series, including K15, K17, K25, K30 and K90, are high-quality pharmaceutical excipients that meet EP and USP standards.
They have excellent hygroscopicity, film-forming properties and complexing abilities, making them very suitable for cosmetic, pharmaceutical and industrial applications.
These products are certified to ensure compliance with international quality and safety standards, providing market access and buyer confidence.
Structure formula:

Appearance: White or yellowish-white powder
CDE register number: K30: F20209990878,K90: F20230000040
Application: K25, K30: binders, film formers, dispersers, stabilisers for oral preparation:
K90: binders,thickeners, stabilisers for oral preparation.
PVP K15 | PVP K17 | PVP K25 | PVP K30 | PVP K60 | PVP K90 | PVP K120 | |
pH | 3.0-5.0 | 3.0-5.0 | 3.0-5.0 | 3.0-5.0 | 4.0-7.0 | 4.0-7.0 | 4.0-7.0 |
K-value | 12.75-17.25 | 15.30-18.36 | 24.0-27.0 | 27.0-32.4 | 54.0-64.8 | 81.0-97.2 | 108.0-130.0 |
Aldehydes (ppm) | ≤500 | ≤500 | ≤500 | ≤500 | ≤500 | ≤500 | ≤500 |
Peroxides (ppm) | ≤400 | ≤400 | ≤400 | ≤400 | ≤400 | ≤400 | ≤400 |
Formic acid (%) | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 |
Hydrazine (ppm) | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 |
NVP (ppm) | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
2-Pyrrolidone(%) | ≤3.0 | ≤3.0 | ≤3.0 | ≤3.0 | ≤3.0 | ≤3.0 | ≤3.0 |
Heavy Metals (ppm) | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
Water (%) | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 |
Sulfated ash (%) | ≤0.1 | ≤0.1 | ≤0.1 | ≤0.1 | ≤0.1 | ≤0.1 | ≤0.1 |
Nitrogen (%) | 11.5-12.8 | 11.5-12.8 | 11.5-12.8 | 11.5-12.8 | 11.5-12.8 | 11.5-12.8 | 11.5-12.8 |
All products comply with Ph. Eur, USP/NF, JP,BP certificate of analysis.
Packed in 25 kg plastic drum or fiber drum, inner lined with PE bags. Special packing is available according to customer's requirement. Preserve in tight containers.
A1: Povidone (PVP K-series) is primarily used as a binder, film-former, and disintegrant in oral preparations. It is suitable for a variety of pharmaceutical applications, including tablets, capsules, and granules, ensuring improved dissolution rates and bioavailability of drugs.
A2: Povidone (PVP K15) has a pH range of 3.0 to 5.0, a K value between 12.75 and 17.25, and a water content of less than or equal to 5.0%. It contains less than 500 ppm of aldehydes and peroxides, and less than 1 ppm of hydrazine.
A3: All Povidone (PVP K-series) products comply with Ph. Eur, USP/NF, JP, and BP certificate of analysis, ensuring they meet international quality and safety standards.
A4: Povidone (PVP K-series) is packed in 25 kg plastic drums or fiber drums, inner lined with PE bags. It should be stored in tight containers to maintain its quality and stability.
A5: Povidone (PVP K-series) offers benefits such as improved dissolution rates, enhanced bioavailability, and increased stability of pharmaceutical formulations.